The 3-Minute Rule for Bacteria Testing
Wiki Article
Get This Report on Bacteria Testing
Table of ContentsThe Of Bacteria TestingRumored Buzz on Bacteria TestingThings about Bacteria TestingNot known Details About Bacteria Testing The Bacteria Testing Statements
Matters at 37o, C have been utilized to indicate faecal contamination in the past, but this is not generally considered to be trustworthy. Many official methods for microbiological water evaluation still depend on standard culture approaches and MF techniques, the time is taken to acquire outcomes, normally 24-48 hours, has focused attention on different fast methods.The major disadvantage of this approach is that it may find non-viable cells and overstate the population, however it seems most likely that QPCR-based approaches will end up being progressively vital in water microbiology, leading to the development of industrial items comparable to those already made use of for food evaluation. Throughout the, traces of the viral RNA was located in sewage at therapy plants in one research executed in The Netherlands.
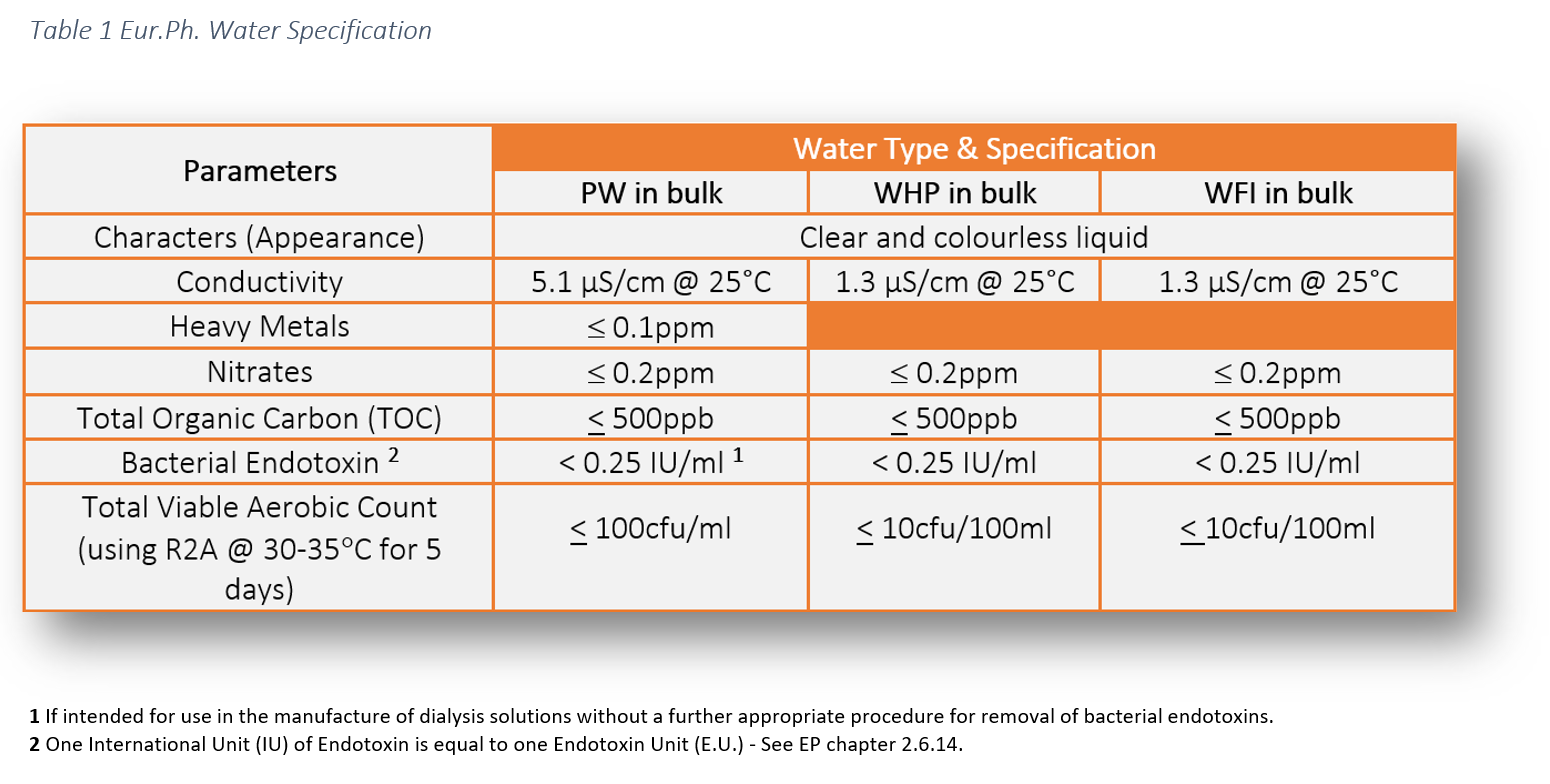
Some Known Incorrect Statements About Bacteria Testing
The filter is after that moved aseptically to the surface area of an agar plate, or an absorbent pad saturated with a suitable discerning tool and incubated. Nests are permitted to create externally of the filter and can be counted and taken a look at straight. MF approaches are fast and very easy to carry out, require little incubator space and can handle huge quantities of water if needed.In regards to tools, MF techniques require appropriate purification device containing a base sustaining a porous disc, on which the filter is placed, and a sterile filter funnel, which can be safeguarded to the base, securing the filter in placement. A range of purification systems are readily offered, including multiple systems in the kind of manifolds, to ensure that more than one sample can be filteringed system at the same time.
Vendors include Millipore, Sartorius and Membrane Layer Solutions. The filter device is attached to an appropriate vacuum cleaner resource to draw the samples through the filter. This may be her explanation a vacuum cleaner line or a stand-alone pump unit. Compact pump devices specifically developed for usage with MF approaches are currently available. An instance is the Millipore EZ-Stream system, which can run the filteringed system sample straight to drain pipes, thus saving time invested clearing and cleaning the waste example containers utilized with find more standard research laboratory air pump.
The Only Guide for Bacteria Testing
45m pore dimension. The filters are generally marked with a grid to aid nest checking.
Even if you do not presume any kind of well water problems, it is essential to check your water to make certain that it is safe to consume. The following table notes the examinations VDH suggests for all private wells also if you do not notice any troubles with your water (Bacteria Testing). Standard Indicators (Potability) The fundamental indicator list covers the most common potential contaminants related to private wells
Herbicides/Pesticides Checking for herbicides and pesticides might be warranted if (a) your well is situated closer than 50 feet from a structure structure that was treated for termites before 1990, (b) significant quantities of herbicides or chemicals have actually been used near your well, or (c) if your well water is discovered to consist of elevated nitrite or nitrate focus and an agricultural resource is presumed.
The Buzz on Bacteria Testing
Your water looks, scents, and tastes fine, however it might not be safe to drink. Lots of Maine wells have way too much arsenic, uranium, radon, or other dangerous chemicals. The only means to know if your water is risk-free to drink is to examine it. 1 in 10 wells in Maine has way too much arsenic, uranium, radon, or various other hazardous chemicals.
The state of Florida does not have a need for well sampling when private homes are marketed. Any licensed drinking water lab should be able to take care of these tests for a practical cost.
Not known Details About Bacteria Testing

Please be conscious that not every laboratory on the list will suit your demands. You might need to call numerous in order to find the appropriate one for you.
Microbial contaminations of food throughout the food production process can occur at any moment. Food putridity is a lot extra typical and most likely when foods are not stored properly, when they are not maintained under heat or in cold/frozen storage space and when there is a lot of moisture. Bacteria Testing. For food handling plants, food assembly line, create distributors, dining establishments and any kind of other food oriented sector it is critically important to do routine food microbiology screening
Report this wiki page